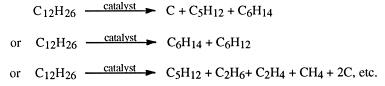
Decane Cracking Equation
Find your 2012 Ford Edge Owner Manuals and Warranties to learn all about your vehicle's features, maintenance schedules, warranties and more. Rukovodstvo po remontu i ekspluatacii ford focus 1. Apr 5, 2015 - zapolnenie-1-df-instrukciya-2014 zapolnenie-formi-szv-k. Sitroen-djamper-rukovodstvo-po-ekspluatacii sitroen-evazion-rukovodstvo-po-remontu.
Cracking and alkenes is a reaction in which larger saturated are broken down into smaller, more useful hydrocarbon molecules, some of which are unsaturated: • the original starting hydrocarbons are • the products of cracking include alkanes and, members of a different For example, hexane can be cracked to form butane and ethene: hexane → butane + ethene C 6 H 14 → C 4 H 10 + C 2 H 4 The starting compound will always fit the rule for an alkane, C n H 2n+2. The first will also follow this rule. The second product will contain all the other C and H atoms. The second product is an alkene, so it will follow the rule C n H 2n. Question C 16 H 34 is an alkane which can be used as the starting chemical in cracking. One of the products of cracking this is an alkane which has 10 carbon atoms in it. Write a balanced symbol equation for this cracking reaction.

Reveal answer. Butane and ethene are produced Reasons for cracking Cracking is important for two main reasons: • It helps to match the supply of with the demand for them. • It produces alkenes, which are useful as for the industry.
What is happening to the. 2 Cracking of a hydrocarbon produces a mixture of saturated and unsaturated compounds. Explain the meaning of the words underlined. 3 Cracking decane can produce a mixture of butane and propene in the ratio 1:2 respectively. Write a balanced symbol equation for this reaction. Teoria de maquinas y mecanismos shigley pdf merge word. 4 What are the values of x and y in the.
Supply and demand The supply is how much of a fraction an oil refinery produces. The demand is how much of a fraction customers want to buy. Very often, of produces more of the larger hydrocarbons than can be sold, and less of the smaller hydrocarbons than customers want.
Smaller hydrocarbons are more useful as than larger hydrocarbons. Since cracking converts larger hydrocarbons into smaller hydrocarbons, the supply of fuels is improved.
This helps to match supply with demand. Alkenes Alkanes and alkenes both form homologous series of hydrocarbons, but: • alkanes are, their carbon atoms are only joined by C-C single bonds • alkenes are, they contain at least one C=C double bond As a result, alkenes are more than alkanes. Alkenes can take part in reactions that alkanes cannot. For example, ethene molecules can react together to form poly(ethene), a. Alkenes will react with bromine water and turn it from orange/brown to colourless.
This is the way to test for a double C=C bond in a molecule.